

What is ANGel-T?
Angiotensin-1-7: A Treatment for Neuropsychological and Memory Impairments Following Moderate to Severe Traumatic Brain Injury.
ANGel-T is a scientific study that aims to explore hoe angiotensin 1-7, a special hormone that can expand blood vessels and reduce inflammation, could possibly be used as a treatment for various neuropsychological issues caused by moderate to severe brain injury (TBI). These issues often include ongoing difficulties with memory, attention, and cognitive functions.

Bellal Joseph, MD, FACS
Under the guidance of Vice Chair of Surgery, Dr. Bellal Joseph, the Division of Trauma, Surgical Critical Care, Burns, and Acute Care Surgery spearheads pioneering research into Angiotensin 1-7's potential in addressing neuropsychological issues post-TBI.
Joseph leads the ANGel-T study, committed to assessing Angiotensin 1-7's impact on TBI-related cognitive challenges, potentially marking a breakthrough in neuroprotective treatment. The collaborative framework across disciplines and the team''s dedication to achieving meaningful results that have the potential to revolutionize the landscape of treating traumatic brain injuries.
Study Aims
AIM 1: SAFETY
Assess the safety and ability to tolerate 21 days of daily subcutaneous (s.c.) Ang-(1-7) administration in individuals with moderate to severe traumatic brain injury (TBI).
AIM 2: EFFICACY
Determine the effects of 21 days of treatment of Ang-(1-7) on:
1. Levels from baseline to follow-up on serum levels of CNS damage biomarkers,
2. Changes in brain MRI volumetrics and changes in cerebral blood flow and BBB, and
3. Hospital length of stay.
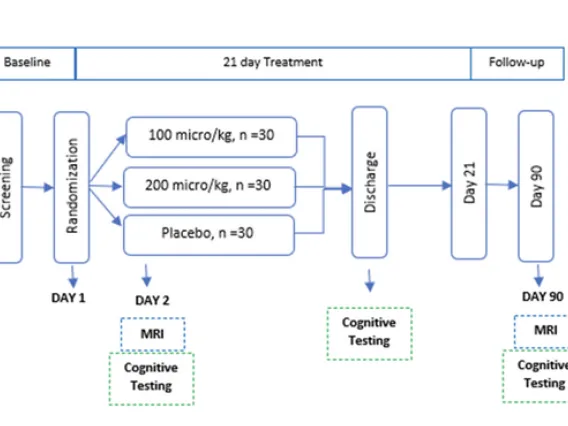
Study Design
Randomized
Placebo-controlled
Double-blind
Three Arms
Ang-1-7 (100 micro/kg) = 30
Ang-1-7 (200 micro/kg) = 30
Placebo = 30
4 Years

Our Preliminary Research
Pre-Clinical
In previous preclinical investigations UArizona researchers have determined that peptides containing angiotensin 1-7 exhibit effectiveness in safeguarding brain function and reducing brain inflammation. Moreover, these medications enhance cerebral blood flow, showcasing significant potential in the treatment of cognitive decline resulting from traumatic brain injury (TBI) and other brain diseases associated with inflammation, such as vascular dementia.

Clinical Trial
The initial doses for our upcoming study on traumatic brain injury (TBI) are determined based on the ongoing proof-of-concept pilot clinical trials that we are currently conducting. These trials involve the use of native Angiotensin-(1-7) to address cognitive impairment and prevent vascular cognitive impairment and dementia (VCID) in cardiac bypass patients. Additionally, we are enrolling patients <IS THERE A LINK?> for a second pilot study focusing on heart failure (HF) patients.
Why is this important?
Clinical trials have examined various neuroprotective agents and approached aimed at managing the neuropsychological and memory impairments resulting from TBI, yet none have exhibited substantial improvements in outcomes. Angiotensin 1-7 may present as the initial effective neuroprotective solution to counter the heightened structural damage and physiological changes in neurological function brought on by TBI.
Approximately 1.7 million individuals endure TBI annually, with prevalent risk factors including falls, particularly among young children and older adults, participation in contact sports, involvement in combat among soldiers, transportation mishaps, and physical abuse. The inflammatory response following TBI is believed to be a fundamental factor influencing the long-term consequences of this condition.
In the pursuit of healing and discovery, the ANGel-T study stands as a beacon of hope, exploring the potential of angiotensin 1-7 to unlock new realms in treating traumatic brain injuries. With each phase, we illuminate pathways to recovery, guided by the spirit of innovation and collaboration. Together, we aim to redefine the future of neurological care, one breakthrough at a time.
Meet Our Team
Image

Bellal Joseph, MD, FACS Principal Investigator Martin Gluck Professor of Surgery Vice Chair of Surgery Division Chief of Trauma, Critical Care, Burns, and Acute Care Surgery University of Arizona College of Medicine - Tucson | Image

Meredith Hay, PhD Co-Investigator Professor, Department of Physiology Professor, BIO5 Institute University of Arizona College of Medicine - Tucson | Image

Theresa Lee Ryan, PhD Co-Investigator Professor, Department of Psychology Professor, Department of Neurology University of Arizona College of Medicine - Tucson |
Image

Todd Vanderah, PhD Investigator Co-Director, MD/PhD Dual Degree Program Chair, Department of Pharmacology | Image

George Alex Hishaw, MD Co-Investigator Assistant Professor, Neurology and Psychiatry Medical Director, Polytrauma at Southern Arizona VA | Image

Nan-Kuei Chen, PhD Co-Investigator Associate Professor Biomedical Engineering |
Chiu-Hsieh (Paul) Hsu, PhD Co-Investigator Associate Professor, Biomedical Engineering |
Robin Carlson, PhD Co-Investigator Clinical Research Manager, Division of Trauma | Image

Abdul Tawab Saljuqi, MD, DrPH Co-Investigator Project Manager Assistant Director for Research, Department of Surgery University of Arizona College of Medicine - Tucson |
This team of experts in various fields, including physiology, pharmacology, psychology, neurology, biomedical engineering, epidemiology, and public health, is working together to explore the role of inflammatory reactions in the long-term effects following TBI.
What Lies Ahead?

Advancing Breakthroughs: Journey Through Clinical Trials and Strategic Partnerships
In our pursuit of a groundbreaking medical breakthrough, we encountered important milestones in the form of clinical trails. Phase 2 involved evaluating the safety and effectiveness of our product, which was administered in two doses to a dedicated group of 180 subjects.
This crucial phase required a significant investment, ranging from $25-$30 million, generously supported by the National Institutes of Health (NIH). Moving on to Phase 3, our focus remained on ensuring safety and effectiveness, but on a larger scale and with a greater financial commitment, ranging from $30-$50 million.
To reach this stage, we formed a partnership with a pharmaceutical company and secured essential venture funding. As we made progress, the final stages leading up to filing a New Drug Application (NDA) and post-market operations demanded intensive effort. It was during this critical phase that we continued to collaborate with the pharmaceutical industry and secured additional venture funding, bringing us closer to making a significant impact in the field of medicine.
Within the intricate web of science and compassion, the ANGel-T study unfolds, driven by a collective commitment to unveil the therapeutic potential of angiotensin 1-7. In this journey of exploration, we aspire to rewrite the narrative of traumatic brain injury treatment, fostering resilience and restoring hope for a brighter, healthier future.
Get In Touch
Bellal Joseph, MD, FACS
University of Arizona College of Medicine - Tucson, Department of Surgery
P.O. Box 245063
Tucson, AZ 85724-6063

